OVERVIEWTriesta Sciences, a unit of HealthCare Global (HCG) Enterprises Limited, is the state-of-the-art, one-stop solution for cancer diagnostics, genomics (next-generation sequencing-based diagnostics), biomarker and translational research, laboratory services, and clinical research services. Based out of Bangalore, Triesta Sciences is an integration of laboratory services, research and development, and clinical research with a focus on innovation, quality, and accuracy for better diagnosis and prognosis of cancer.
Triesta Reference Laboratory is the leading CAP- and NABL-accredited high-end oncology diagnostic laboratory service provider in India, offering a comprehensive range of routine to highly specialised diagnostic tests for hospitals, and medical institutions within India and overseas.
Key Departments
Histopathology
The histopathology laboratory of Triesta Sciences, Bangalore, is the reference lab for the HCG network. It is spread over an area of 1800 sq. ft. and is well equipped with state-of-the-art equipment. This lab serves as the centre of excellence for histopathology throughout the HCG network. The lab receives over 20,000 histopathology specimens a year from its network hospitals as well as outside referrals.
The laboratory provides basic histopathology services, immunohistochemistry, cytology, frozen sections, and digital pathology services. There are over 180 immunohistochemistry markers of diagnostic, prognostic, and therapeutic significance, including markers like ALK (D5F3), PDL1, MSI, etc., supporting the practice of precision oncology. The laboratory also provides LBC services.
The laboratory is the first 100% digital pathology laboratory in India for primary diagnosis and implementation of AIML-based breast biomarker algorithms for clinical use.
The laboratory supports national and international collaboration for remote reporting of histopathology cases. Triesta Sciences is equipped with state-of-the-art equipment in histopathology from reputed manufacturers like Leica, Thermo Fisher, and Sakura. The laboratory has an FDA-approved, fully automated immunohistochemistry machine for all its IHC markers. HCG is equipped with a US FDA-approved digital pathology scanner from Philips, with its image management system and central portal server.
HCG has been recognised and awarded as the first 100% digital pathology laboratory for primary diagnosis in India since October 2018.
The laboratory has completely embraced a digital workflow ensuring 100% patient safety, accurate staging, and grading of tumours, saving logistics costs, collaboration, and improved efficiency. It is the first laboratory in the country to implement an IVDCE-approved, artificial intelligence machine learning-based (AIML) algorithm for objective interpretation of breast biomarkers.
The laboratory has been accredited by the College of American Pathologists (CAP) and NABL based on ISO 015 189 2012 standards, for the last 8–10 years.
Our histopathology laboratory is the first in the country to get these accreditations with digital pathology and an image-based artificial intelligence machine learning breast biomarker algorithm in its scope of accreditation.
There are 12 histopathologists in the HCG network. The Bangalore centre has three pathologists who practice organ-specific sub-speciality histopathology reporting. They are well-qualified and highly experienced in the field of oncopathology. They are also trained in generating reports for digital pathology images (WSIs), remotely. There are three senior residents who are post-MD, doing their fellowship in tumour pathology, as well as eight junior DNB residents. There are seven highly skilled histopathology and immunohistochemistry technicians, along with a digital pathology coordinator. This technical team is well supported by customer care and front office executives.
The pathologists in HCG are experts in organ systems and practice subspeciality organ-specific reporting. Internal team discussions supported by a vast gamut of IHC, and genomics ensure a strong, objective, evidence-based diagnosis. This ensures the practice of precision oncology. The pathologists regularly participate in clinical case discussions and MDTs, thereby contributing to efficient patient management.
Digital Pathology
A Revolution in the Field of Cancer Diagnosis and Treatment
Triesta Sciences, a division of HealthCare Global Enterprises, was the first referral laboratory in India to use digital pathology for primary diagnosis in histopathology. With a network of 22 oncology centres across India and abroad and a talented pool of histopathologists, the need for integrating all the centres to provide a uniform standard of reporting was envisioned by Dr. BS Ajaikumar, Executive Chairman, HCG. This vision became a reality with the implementation of digital pathology in the HCG network. Through organ-specific reporting by subspecialist histopathology experts, this approach aims at delivering superior diagnostic support within a short turnaround time, saving on logistics.

Digital Pathology Set-up
Since October 2018, digital images and whole slide images generated on an FDA-approved digital pathology scanner have been the mode of primary diagnosis in histopathology in the Triesta referral lab in HCG, Bangalore. The entire caseload of the day is digitised providing whole slide images that provide tools for accurate measurements for staging and quantification of tumours, counting mitoses, interpreting lymphovascular and perineural invasion accurately, along with identification of isolated tumour cells, micro-metastasis or macro-metastasis, major or minor extracapsular extension, etc. The pathologists are extensively trained in reporting on whole slide images and have a diagnostic concordance of 99.7%.
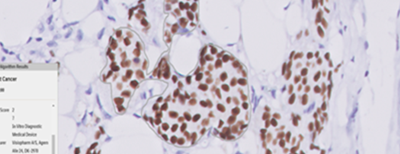
Breast Cancer Biomarker Reporting
HCG is also the first laboratory in the country to validate and implement an image analysis-based artificial intelligence algorithm for breast biomarker interpretation (ER, PR, HER-2, and Ki67) with 97.7% to 100% accuracy, for clinical use. Triesta participates in the CAP proficiency testing programs for these WSI and image-based algorithms with 100% concordance results.
- Report generation by organ-specific expert pathologists in oncopathology.
- National and international collaboration for primary diagnostic services.
- Accurate quantification, staging, and grading of tumours.
- Faster turnaround time.
- Reduced logistic costs.
- Regular case discussions and enhanced quality diagnostic support.
- Objective interpretation of breast biomarkers through AI-based, IVCED-certified algorithms.
HCG has also provided a platform for the development and validation of several artificial intelligence-machine learning-based algorithms for biomarker interpretation in pathology and radiology through the enormous digital images that are generated. The institute aims at developing AIML algorithms and validation in oncology, computational pathology, radiogenomics, and other integrated clinical services.
With a huge repository of more than 1.5 lakh whole slide images that are produced every year, HCG has a huge organ-specific biorepository that is available for medical education and biomarker research.
- Basic histopathology services
- Immunohistochemistry
- Cytology
- Frozen section
- Digital pathology services
Molecular Diagnostics
The Department of Molecular and Clinical Genomics is a state-of-the-art genomics centre offering comprehensive molecular diagnostics based on technologies like PCR, cytogenetics, FISH, and next-generation sequencing to all patients to improve therapy selection, predict response and prognosis, screen for hereditary cancer and predict predisposition and risk.
Triesta Sciences is the best place to study the genomic profiling data of tumour samples and provide robust analysis and biological interpretation of large data sets using analytics and interpretation platforms. Along with this and the constant addition of new technologies, such as liquid biopsy, this department is geared up to be one of the most advanced labs globally.
With the information on current and emerging biomarkers, we are also working with pharmaceutical companies to identify and validate new targets based on the genomic signature for drug discovery and development, the discovery of tumour-agnostic biomarkers, drug repurposing, and the enrollment of patients for more enriched clinical trials.
The Department of Molecular and Clinical Genomics is headed by Dr. Mithua Ghosh, director – clinical diagnostics, who is an accomplished cancer biologist with over 20+ years of academic and corporate experience and expertise in the fields of cancer biology, molecular pathology and genomics, diagnostics, translational research, stem cell research, and target-based drug discovery.
She is assisted by competent team leads in each division. The department comprises a multi-talented team of PhDs, post-doctorates, MDs, pathologists, molecular biologists, geneticists, and genetic counsellors.
Factors that make Triesta’s approach to cancer diagnosis unique:
- Strong collaboration with physicians.
- Committed to the highest quality solutions.
- Comprehensive test range, from simple to highly specialised.
- Innovative diagnostic platforms and tests.
- A multidisciplinary approach to diagnosis.
Services Offered:
- Molecular pathology services: PCR-based single gene tests for tumour profiling
- Cytogenetics: karyotyping and FISH
- Genomics services
- Liquid biopsy services
- HLA and chimerism
- Translational research services
Haematology, Clinical Pathology and Flow Cytometry
The haematology department is equipped with the latest CBC and coagulation analysers and provides round-the-clock services. The laboratory has two state-of-the-art ten-colour flow cytometers and offers advanced flow cytometry services for investigations and management of various haematological conditions. It is one of the few labs in the state that offers reliable minimal residual disease testing for blood cancers.
Services Offered:
- Haemogram and peripheral blood reporting
- Basic and advanced coagulation workups for various bleeding and clotting disorders
- Bone marrow aspiration and trephine biopsy reporting
- Immunohistochemistry on bone marrow biopsies
- CD34/ stem cell enumeration for bone marrow transplants
- Acute leukaemia immunophenotyping
- Immunophenotyping for chronic lymphoproliferative disorders and myeloma
- Minimal residual disease testing for various leukaemias
- Flow cytometry studies for PNH
- Investigations for haemolytic and other anaemias
- Various clinical pathology services like urine and stool analysis, urine for dysmorphic RBCs
Biochemistry
The lab houses a full-fledged biochemistry department that has a talented pool of specialists who provide diagnostic laboratory services to outpatients and inpatients. This department is committed to maintaining the highest quality of performance using the latest technology and equipment that enables us to deliver rapid and reliable results to patients in the shortest turnaround time.
The biochemistry department, along with the other lab departments, is interconnected to the hospital by a Laboratory Information System (LIS) for quicker and more accurate generation of reports. Samples are barcoded and fed into the equipment, which automatically performs the tests indicated by the bar code. The test results obtained are immediately transferred online to the doctor’s desk after verification by a qualified consultant or a senior technician who has undergone training as an internal auditor from NABL.
Routine biochemistry and specialised tests like hormones, tumour markers, vitamins, and therapeutic drug monitoring are carried out every day.
Microbiology
Our microbiology department provides robust diagnostic services, along with stringent hospital infection control, with most of the microbiology tests done in-house as per NABL and CAP protocols with defined turnaround times.
Cultures are done on an automated system that is near-accurate. Quality assurance is followed from the time the sample is received until the reports are generated.

